electron withdrawing groups|electron donating ability : Pilipinas • Electron-donating group Tingnan ang higit pa Czech Mega Swingers Channel 214,871,381 214.9M video views 214.9M views 65k. Add to friends. 65k. Add to friends. The largest swingers event in the world, full of amazing Czech amateur pairs - no fakes and no script! Each evening is full of fresh girls and we let everything happen casually and without any restraints. You'll see a truly wide .
PH0 · inductive electron withdrawal
PH1 · electron withdrawing inductive effect
PH2 · electron withdrawing groups list
PH3 · electron withdrawing effect
PH4 · electron donating effect
PH5 · electron donating and withdrawing groups
PH6 · electron donating ability
PH7 · diels alder stereochemistry
PH8 · Iba pa
We look at 7-time Mr. Olympia Phil Heath's basketball years and how they fueled his legendary bodybuilding career. THE BARBELL Bodybuilding, Strength, and . His gift was making muscle, and his 5’9” height, such a liability in basketball, was ideal for bodybuilding success. In a reversal of his college basketball years, he was a genetic .
electron withdrawing groups*******An electron-withdrawing group (EWG) is a group or atom that has the ability to draw electron density toward itself and away from other adjacent atoms. This electron density transfer is often achieved by resonance or inductive effects. Electron-withdrawing groups have significant impacts on . Tingnan ang higit pa
Effects on Bronsted acidityElectron-withdrawing groups exert an "inductive" or "electron-pulling" effect on covalent bonds. The strength of the electron . Tingnan ang higit paElectron-withdrawing groups are the opposite effect of electron-donating groups (EDGs). Both describe functional groups, however, . Tingnan ang higit paelectron withdrawing groups electron donating ability• Electron-donating group Tingnan ang higit pa The more electron-rich the aromatic ring, the faster the reaction. Groups that can donate electron density to the ring make EAS reactions faster. If a substituent . Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. Examples of electron withdrawing .Learn the definition, examples and effects of electron withdrawing groups (EWG) and electron donating groups (EDG) in organic chemistry. Find out how they influence .
In electrophilic aromatic substitution reactions, existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing effect on positional isomer of the products that are formed. An electron donating group (EDG) or electron releasing group (ERG, Z in structural formulas) is an atom or functional group that donates some of its electron density into a conjugated π system viaCarbonyl groups are electron-withdrawing by inductive effects, due to the polarity of the C=O double bond. It is possible to demonstrate in the laboratory that carbocation A below is more stable than carbocation B, .
electron withdrawing groupselectron withdrawing substituents increase the Lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease Lewis acidity by making sites less .Learn how an electron withdrawing group (EWG) to a benzyl cation destabilises it, and how the position of the EWG affects the stability. Watch a video created by Shahzad Karim and see .
Learn how substituents affect the reactivity of aromatic rings in electrophilic substitutions. This web page is part of a free textbook on organic chemistry, but it has a glitch and .Learn Electron Withdrawing Groups with free step-by-step video explanations and practice problems by experienced tutors.
EWG, electron-withdrawing group. Full size image. Radical philicity and HAT chemistry. Radical philicity can influence the outcome of many types of organic reactions, .Because Lewis acid-base reactions involve electron donation and acceptance at particular sites, substituent groups which alter the electron density at a site through inductively donating or withdrawing electron .electron donating ability In general, Diels-Alder reactions proceed fastest with electron-withdrawing groups on the dienophile (diene lover). Ethylene reacts slowly while propenal, ethyl propenoate, and other molecules .
Effect of an electron withdrawing group in a benzyl cation. In this video, we see that an electron withdrawing group to a benzyl cation destabilises it. However adding it at ortho- and para- destabilises .As described earlier in this section, hydroxyl, alkoxyl, and amino groups have a strong, electron-donating resonance effect that outweighs a weaker electron-withdrawing inductive effect. When phenol is nitrated, for instance, reaction can occur either ortho, meta, or para to the –OH group, giving the carbocation intermediates shown in Figure .

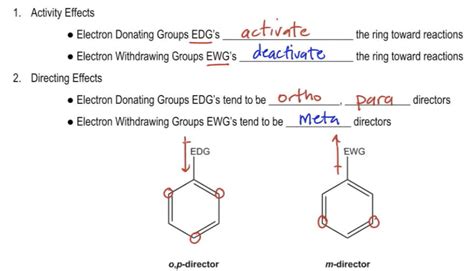
Okay, Whereas many directors electron withdrawing groups, right, they pull electrons out of the ring, so they're gonna tend to add in the meta positions. Okay, I forgot to draw the Dye poll of the electron donating is gonna push electrons into the ring. It directs towards Opie, whereas electron withdrawing groups direct towards the meta positions. In Nucleophilic Aromatic Substitution, an electron-poor aromatic ring is attacked by a nucleophile, resulting in a substitution reaction. The reaction proceeds through a negatively charged (carbanion) intermediate. The reaction is accelerated by the presence of electron-withdrawing groups on the aromatic ring.Here are some general pointers for recognising the substituent effects: The H atom is the standard and is regarded as having no effect. Activating groups increase the rate. Deactivating groups decrease the rate. EDG = electron donating group. EDG can be recognised by lone pairs on the atom adjacent to the π system, eg: -OCH 3.
rom、レトロゲーム、アーケードゲーム機に関する最新情報を入手するのに最適なサイトです。無料ゲームをダウンロードして、プレイステーション、psp、ゲームボーイアドバンス、ニンテンドー64、スイッチ、wii、pc、ドリームキャストなどのromと .
electron withdrawing groups|electron donating ability